Just accepted in JASMS, we argue in this critical insight that in order to enable the development of new-generation CCS […]
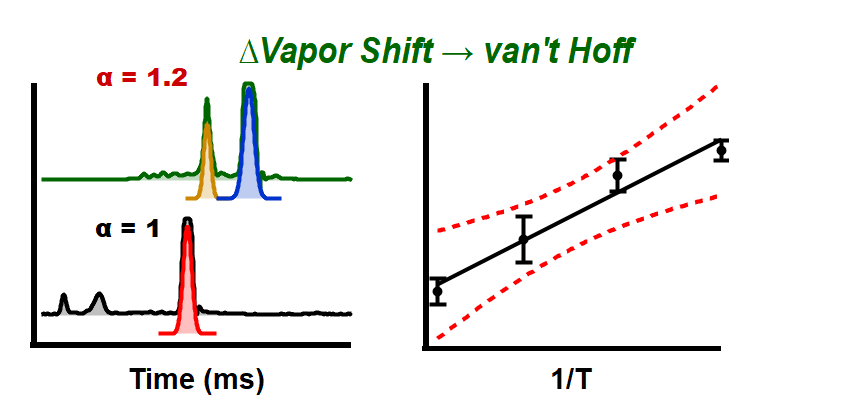
Just accepted in JASMS: Vapor assisted mobility shift measurements were made with atmospheric pressure drift-tube ion mobility–mass spectrometry (IM–MS) to […]
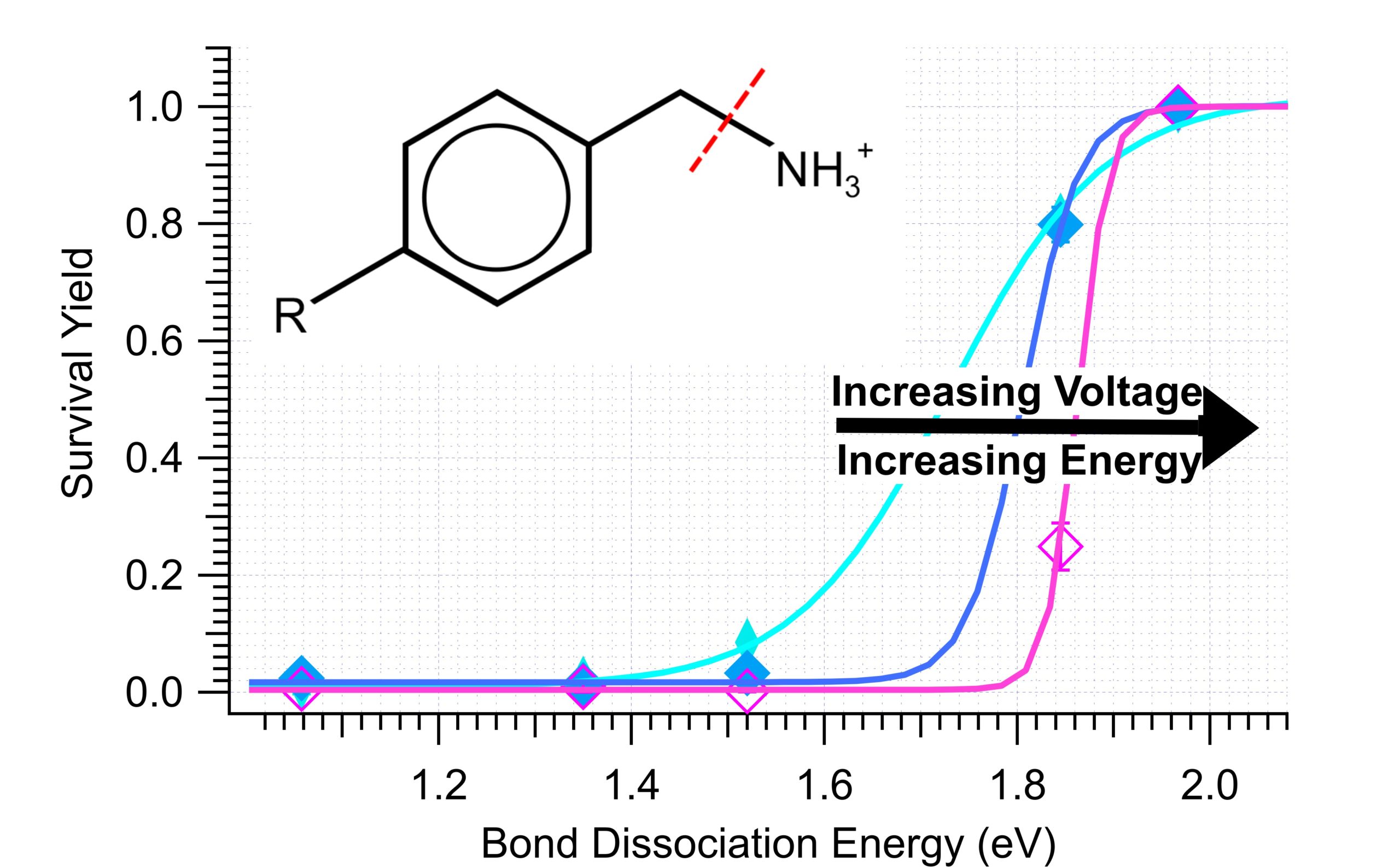
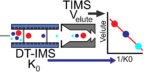
Just accepted in JASMS, we evaluate of TIMS source conditions to determine an effective energy for different voltages applied to […]
Just published in International Journal for Ion Mobility Spectrometry, we compare the ability of the tri-state ion shutter (3S-IS) to the […]
Our latest manuscript was just accepted in Analytical Chemistry. We use a set of standards to discuss the implications of […]
Just accepted in Analyst, we’ve improved on using our tri-shutter grid ion gates by implementing the tri-state pulsing to increase […]
While trapped ion mobility spectrometry (TIMS) provides excellent separation capability as an ion mobility technique, one major drawback is the […]
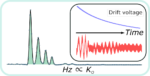
In a standard single averaged, drift tube ion mobility spectrometry (IMS) experiment, typically less than 1% of the ions are […]
Previously, we reported an approach to quantify the energetics of association between neutral drift gas modifiers and two common chemical […]
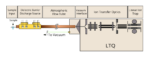
Following our initial experiments using ion trap mass spectrometry and atmospheric flow tube sampling (AFT-MS) for alkylphosphonic acid detection, we […]
Atmospheric flow tube-mass spectrometry (AFT-MS) first emerged in 2012 as an ambient vapor sampling technique developed by Ewing et al. […]
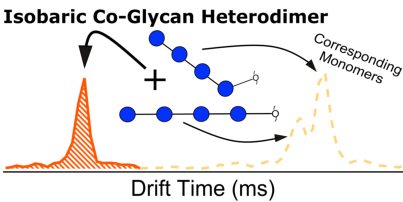
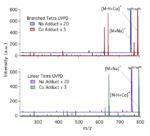
Continuing along our previous work to characterize certain cationized glycans with a variety of metals and with cobalt to enhance […]
Ultraviolet photodissociation (UVPD) has had its application directed primarily at biomolecule analysis, but with a common drawback of low fragment […]
Using a combination of Fourier transform ion mobility and ion trap mass spectrometry Kelsey demonstrated the degree to which different metal […]