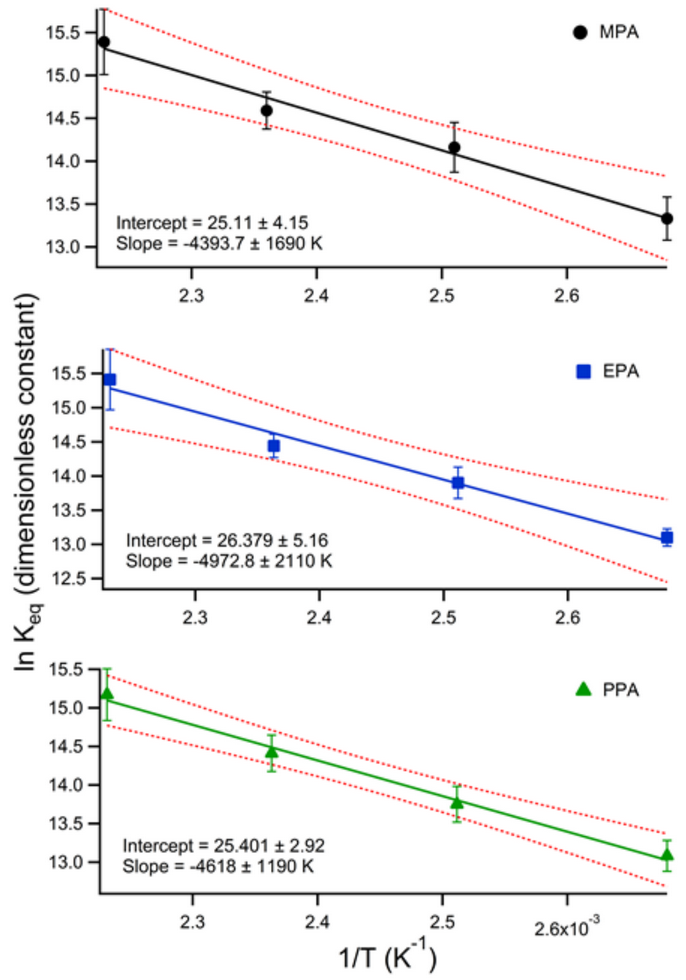
Previously, we reported an approach to quantify the energetics of association between neutral drift gas modifiers and two common chemical warfare agent degradation species by a combination of experimental drift time shift data as well as computational modeling in collaboration with Chris Hogan at the University of Minnesota. As a continuation of this research in conjunction with Dr. Hogan, we are pleased to report the acceptance of our article for publication in The Journal of Physical Chemistry A entitled, “Deducing Proton-Bound Heterodimer Association Energies from Shifts in Ion Mobility Arrival Time Distributions.” This report describes the Gibbs free energy, enthalpy, and entropy changes as propanol is dimerized with a homologous series of alkylphosphonic acids (methyl-, ethyl-, and propylphosphonic acids) and its conformation with the Kelvin-Thomson-Raoult model.

Comments are closed